Get ready for full ISO 15189 and ISO 20658 compliance
End-to-End Sample Tracking for Preanalytics.
We’ve created a practical guide to help labs meet ISO 15189 requirements.
Sending this form I agree to processing of my data to contact me.











ISO 15189
Audit-Ready Data
ISO 20658
Audit-Ready Data
- Made, stored and encrypted in Europe
- Up & Running in 2 weeks
- Hardware agnostic, no big invest upfront needed
- Integrated with any kind of LIS, middleware or standalone
- No change in daily operations whilst bringing light to preanalytical data
- Significant lab TAT reduction
- Tailored to every user group (phlebotomists, courier drivers, lab managers, lab staff)
- Modular setup - take just what you need
- Solves measurement uncertainty ISO 15189
Are Preanalytics Holding You Back from ISO 15189:2022 Compliance?
The latest version of ISO 15189, the ISO 15189:2022, will be enforced on Dec 5, 2025. Laboratories that want to stay compliant with the highest industry standards need to get certified to the newest version of ISO by this December.
If you are currently in the process of renewing or getting from scratch your ISO 15189:2022 or ISO 20658:2023 certification and are looking into requirements for the preanalytical stages of your samples (collection, transportation, sample receipt), you probably:
- Are immersed in a lengthy and complex requirement screening, trying to fulfil everything quickly whilst not doing significant changes to your lab processes and staff
- Have your lab staff very busy during the next months with the certification, significantly adding to their workload
- Want to keep and improve efficiency in your processes, all while complying with the increasing need of keeping and handling data.
- Are thinking of implementing a solution to solve the requirements regarding sample preanalytics, but you don’t want to disrupt daily operations
- Are reluctant to add yet another tool to your existing software suite that most likely does not integrate with any of your processes and tools
- Know that looking for a compliant and trustworthy solution is time-consuming, with many options available on the market and limited time resources
- Need a solution up and running quickly, ideally within a few weeks
- Want to stay innovative and compliant while keeping your budget.
S4DX helps you navigate these challenges efficiently, ensuring a smooth transition to ISO 15189:2022 compliance for all preanalytical stages, all while boosting your efficiency and keeping your day-to-day business running: Get S4DX and boost your efficiency :
- Quickly: digitize your sample collection, transportation, temperature and location tracking, and sample receipting processes online within two weeks including installation and user training
- Effectively: Generate fully ISO 15189:2022 compliant data without having to disrupt your laboratory dynamics. Our open software, available at any kind of PC or phone, and our agnostic hardware, ready for every kind of sample container and box, fits in any process. We adapt to you, not you to us.
- Secure: Your data will be safely stored in EU servers, fully encrypted and compliant with the highest security standards (e.g. HIPAA and ISO 27001). S4DX manufactures entirely in Europe.
- Cost-efficient: Open architecture and no bulky hardware, together with streamlined onboarding and installation processes allow us to provide best-class products fitting in every lab budget.
- Innovative & time-saving: Make the most out of your data and processes, and more. Position yourself as the leading lab in your market and save time in your day-to-day activities by bringing light to your preanalytics workflows.
- End-to-end sample tracking: from sample pre-collection and collection, going to transportation and receipt - we get you covered through the complete sample preanalytics workflow, not just single parts.


S4DX can help you comply with following ISO 15189:2022 requirements
Sample Pre-collection | Collection | Transport | Receipt

7.2.4.2 Sample pre-collection activities
7.2.4.4. Sample collection
7.2.5. Sample transportation
7.2.6.Sample receipt
Laboratories are required to provide information and instructions for pre-collection activities with sufficient detail to ensure that the integrity of the sample is not compromised, including:
- Preparation of the patient (e.g. instructions to caregivers, sample collectors and patients)
- Type and amount of the primary sample to be collected with descriptions of the containers and any necessary additives, and when relevant the order of collecting samples (order overview and preanalytical guidance)
- Special timing of collection
- Provision of clinical information relevant to, or affecting sample collection, examination performance or result interpretation (e.g. history of administration of drugs)
- Link patient/sample for unequivocal identification of the patient, when several samples from the same patient are to be collected
To ensure safe, accurate and clinically appropriate sample collection and pre-examination storage, labs have to provide:
- Verification of the identity of the patient from whom a primary sample is collected.
- Verification recording that the patient meets pre-examination requirements (e.g. fasting status, medication status).
- Collection of primary samples with descriptions of the primary sample containers, as well as the order of sample collection.
- Unequivocal link sample/patient with the patients from whom samples are collected.
- Recording of the identity of the person collecting the primary sample and the collection date and time.
- Requirements for separating or dividing the primary sample* *(if secondary sample has same barcode).
Tracking of storage conditions* before collected samples are delivered to the laboratory *(temperature, shock)
To ensure the timely and safe transportation of samples, the laboratory shall provide instructions for:
- Ensuring the time between collection and receipt in the laboratory is appropriate for the requested examinations.
- Maintaining* the temperature interval specified for sample collection and handling *(S4DX “Monitors” temperature).
- Any specific requirements to ensure integrity of samples.
- If the integrity of a sample has been compromised and there is a health risk, the organization responsible for the transport of the sample shall be notified immediately and action taken to reduce the risk and to prevent recurrence.
7.2.6.1 Sample receipt procedure
The laboratory shall have a procedure for sample receipt that includes:
- The unequivocal traceability of samples by request to a uniquely identified patient and when applicable, the anatomical site
- Criteria for acceptance and rejection of samples* (through S4DX sample flags)
- Recording the date and time of receipt of the sample
- Evaluation of received samples, by authorized personnel, to ensure compliance with acceptability criteria relevant for the requested examinations
- Instructions for samples specifically marked as urgent
- Ensuring that all portions of the sample shall be unequivocally traceable to the original sample
7.2.6.2 Sample acceptance exceptions
The laboratory shall have a process that considers the best interests of the patient in receiving care, when a sample has been compromised due to
- Incorrect patient or sample identification
- Sample instability due to, for example, delay in transport
- Incorrect storage or handling temperature
- Inappropriate container(s) and
- Insufficient sample volume
When a compromised clinically critical or irreplaceable sample is accepted, after consideration of the risk to patient safety, the final report shall indicate the nature of the problem.
Valid For:




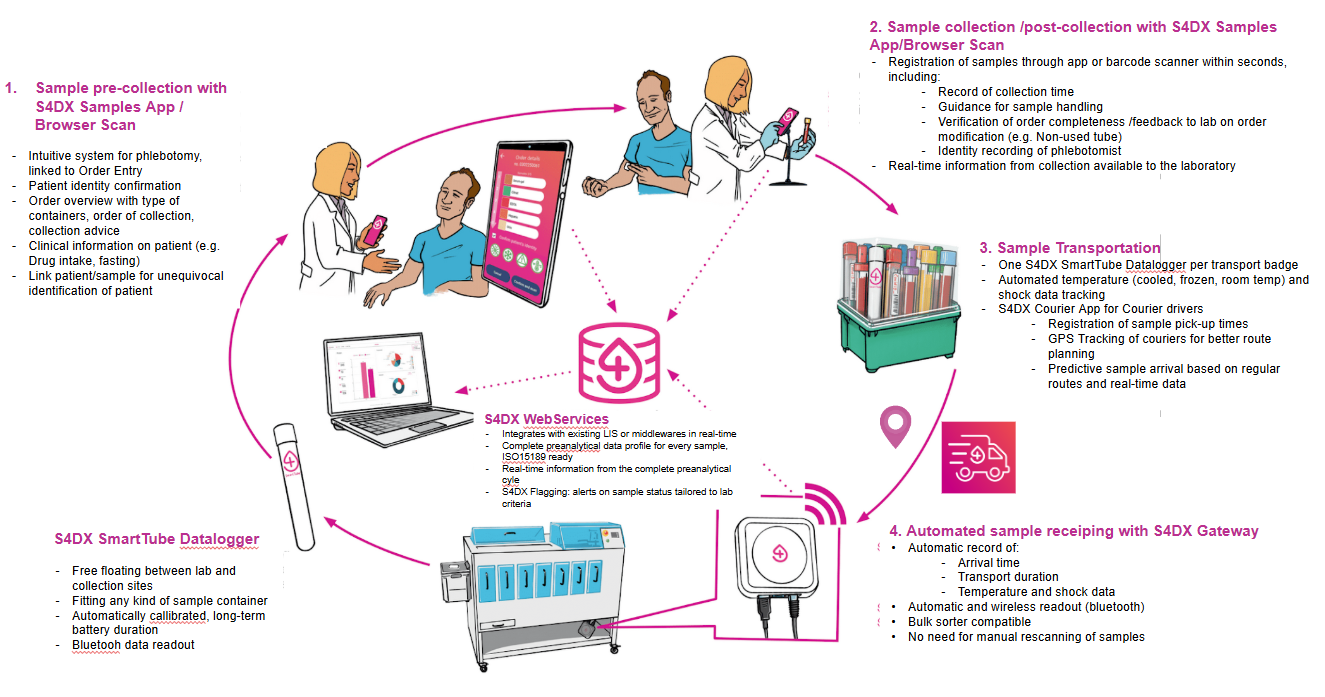
S4DX End-to-end sample tracking for preanalytics, from pre-collection up to lab receipt
- Software-based, either in a web-based browser application with a barcode scanner, or an Android/iOS mobile app.
- Hardware agnostic- S4DX SmartTube Datalogger fits in every kind of sample container.
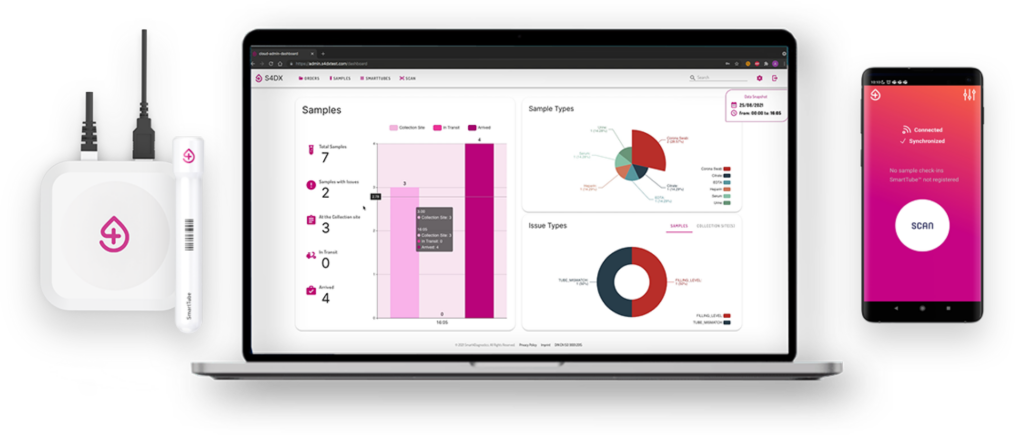
- Powerful insights tailored to each user group (phlebotomist, lab staff, lab manager, courier driver).
- Data storage and encryption according to ISO 15189 criteria.
- S4DX Flagging adjusted to your lab criteria. Set up your own alerts and tolerance parameters.
© 2025 S4DX. All Rights Reserved | Privacy Policy | Imprint | DIN EN ISO 9001:2015 | DIN ISO/IEC 27001:2022